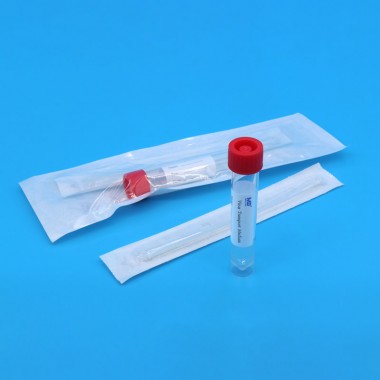
The Virus Transport Media Kit (Inactivated Type) is a ready-to-use solution designed for the safe collection, transport, and storage of clinical specimens suspected of containing viruses such as SARS-CoV-2, Influenza, RSV, and other respiratory pathogens. The kit includes a high-quality inactivated virus transport medium (VTM) that deactivates viruses upon contact, minimizing biosafety risks during sample handling and transportation.
Each kit comes with a sterile nylon flocked swab optimized for efficient sample collection and elution. The inactivated medium preserves nucleic acids (RNA/DNA), ensuring sample stability for downstream molecular analysis such as RT-PCR and qPCR.
Product Parameters| Product Name | Virus Transport Media Kit/VTM Kit/Disposable Virus Sampling Kit/Influenza Virus Sampling Kit |
| Model Number | MVTM-10A |
| Brand Name | MEIDIKE GENE |
| Tube Material | Medical Grade PP |
| Medium Type | Inactivated |
| Swab Type | nylon flocked swab |
| Medium Volume | 3ml/OEM |
| Tube Size | 10ml |
| Shelf Life | 2 Years |
| Certification | CE,FDA,CFDA,TGA,SFDA,ISO13485 |
| OEM/ODM | Available |
FeaturesInactivated medium: Rapidly inactivates viruses to ensure biosafety for healthcare professionals.
Nylon flocked swab: Provides efficient sample collection and elution.
Leak-proof tube: Screw cap design prevents spillage and contamination.
Individually packaged: Ensures sterility and ease of use in clinical settings.
User-friendly design: Ready-to-use format for efficient sample collection and transport.
ApplicationsSample collection for SARS-CoV-2 (COVID-19), Influenza, RSV, and other respiratory virus testing
Molecular diagnostic applications including RT-PCR and qPCR
Use in hospitals, clinics, mobile testing units, laboratories, and customs inspection
PackagingIndividual pacakge.
Custom packaging available upon request.