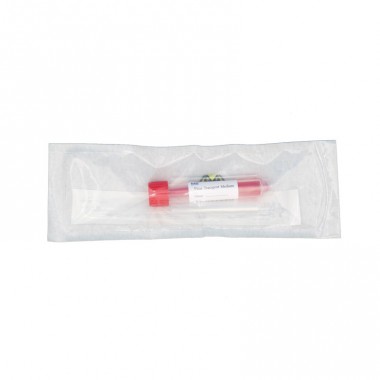
MEIDIKE GENE Viral Transport Media Kit is designed for the collection, preservation, and transportation of clinical specimens containing viruses, including SARS-CoV-2, influenza, and other respiratory pathogens. Each kit includes a 10ml leak-proof screw-cap tube pre-filled with 3ml of non-inactivated viral transport medium, along with a sterile nylon flocked nasopharyngeal swab. The kit ensures high sample integrity and is individually packaged for safe and convenient use.
Components
The VTM Kits include the following components:
- Viral transport medium (VTM)
- nasopharyngeal Swab
- Transport tube
- biohazard bag (optional)
FeaturesNon-inactivated medium: Maintains viral viability for downstream molecular testing.
Nylon flocked swab: Provides efficient sample collection and elution.
Leak-proof tube: Screw cap design prevents spillage and contamination.
Contents : 10ml PP Tube (3ml Non-inactivated medium), 1 flocked nasopharyngeal swab, 1 biosafety bag.
Individually packaged: Ensures sterility and ease of use in clinical settings.
User-friendly design: Ready-to-use format for efficient sample collection and transport.
Product Parameters
| Product Name | VTM Kit/Disposable Virus Sampling Kit/Virus Transport Medium/Virus Sampling Tube/Virus Sampling Kit for Covid |
| Model Number | MVTM-10A |
| Brand Name | MEIDIKE GENE |
| Material | Medical Grade PP, Nylon |
| Liquid Type | Non-inactivated |
| Swab Type | Oral or Nasal |
| Liquid Volume | 3ml/OEM |
| Size | 10ml |
| Shelf Life | 2 Years |
| Application | Collection and transport of viral specimens from patients |
| Certification | CE,FDA,CFDA,TGA,SFDA,ISO13485 |
| OEM/ODM | Acceptable |
Instructions for Use
1. Open the VTM Kit and remove the swab and transport tube.
2. Using the swab, collect the specimen from the appropriate site.
3. Insert the swab into the transport tube and break off the handle at the scored line.
4. Tighten the cap on the transport tube.
5. Label the transport tube with the patient's name, date of collection, and specimen type.
6. Place the transport tube in the provided biohazard bag and seal.
7. Place the biohazard bag in a secondary container for transport.