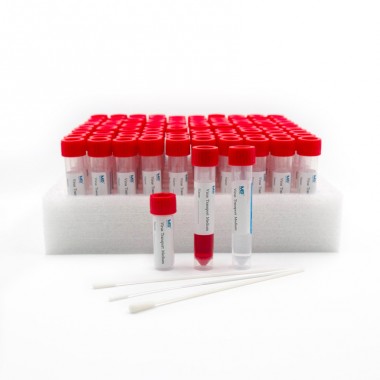
MEIDIKE GENE® VTM Kits are a set of products designed to collect and preserve clinical specimens for laboratory testing. The kits contain all the necessary components to ensure the safe and efficient collection, transportation, and processing of specimens.
Components
The VTM Kits include the following components:
- Viral transport medium (VTM)
- Swab
- Transport tube
- Label
Product Parameters
| Product Name | VTM Kit/Disposable Virus Sampling Kit/Virus Transport Medium/Virus Sampling Tube/Virus Sampling Kit for Covid |
| Model Number | MVTM |
| Brand Name | MEIDIKE GENE |
| Material | Medical Grade PP, Nylon |
| Liquid Type | Inactivated or Non-inactivated |
| Swab Type | Oral or Nasal |
| Liquid Volume | 6ml/OEM |
| Size | 5ml/10ml/15ml/30ml |
| Shelf Life | 2 Years |
| Application | Bio Sample Colllection/For sampling collection,transportation and storage |
| Certification | CE,FDA,CFDA,TGA,SFDA,ISO13485 |
| OEM/ODM | Acceptable |
Instructions for Use
1. Open the VTM Kit and remove the swab and transport tube.
2. Using the swab, collect the specimen from the appropriate site.
3. Insert the swab into the transport tube and break off the handle at the scored line.
4. Tighten the cap on the transport tube.
5. Label the transport tube with the patient's name, date of collection, and specimen type.
6. Place the transport tube in the provided biohazard bag and seal.
7. Place the biohazard bag in a secondary container for transport.
Precautions
VTM Kits are intended for in vitro diagnostic use only. The kits should not be used after the expiration date or if the packaging is damaged. The kits should only be used by trained healthcare professionals. The specimens collected using the VTM Kits should be handled in accordance with standard laboratory procedures and appropriate biosafety measures.